-40%
10.1"Touch LCD Spirometer Pulmonary Function Lung Volume espirómetro SP100A Wifi
$ 441.4
- Description
- Size Guide
Description
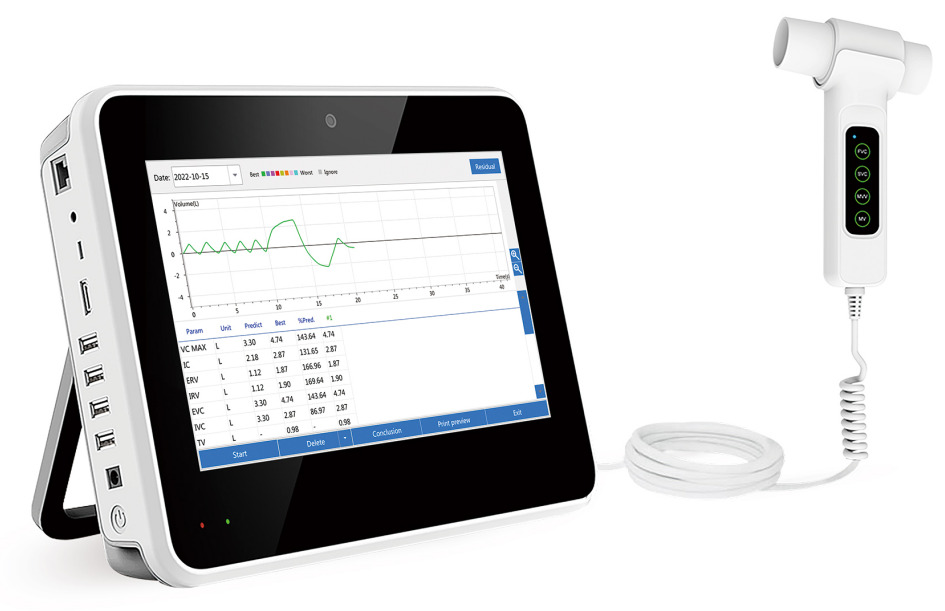
CONTEC SP100AIntroduction
The Spirometer is a portable device for testing pulmonary function, it adopts differential pressure acquisition principle to measure over 100 parameters related to FVC, VC, MVV and MV functions. It can be used in many scenarios, such as hospital, clinic, family, MVV and provocation test before / after medication, etc. which can provide the test results of pulmonary function for the users and the basis for the medical staff to make diagnosis.
Function
Differential pressure detection technology, with a professional lung function software, which can measure over 100 parameters related to FVC, VC, MVV and MV functions.
Measure inspiratory and expiratory parameters, real-time curve display.
BTPS correction function, measure temperature, humidity and barometric pressure automatically.
Quality control function, automatic QC rating.
Calibration control function: volume calibration, three flows verification.
Display the optimal value from many measurements.
The tester condition can be reflected by the ratio of the measured value and the predicted value.
Flow-volume and volume-time curve.
Data memory, delete and information review functions.
Trend chart display.
Prompt functions for over-limit of volume and flow.
Rechargeable lithium battery, with charging prompt.
Battery power indication.
Built-in multiple predicted values.
Support bronchial dilation / provocation test.
Pulmonary function assessment.
Follow-up questionnaires function.
Patient information can be entered, managed and queried.
The external printer can be connected to print and output the test report.
10.1" capacitive touch screen.
Wi-Fi function.
Voice prompt function.
Buy safe products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
International Buyer--Please note:
Import duties,Taxes are not included in the item price.These charges are buyers' responsibility.
Return:
We offer 60 days return policy.If the item has any defect,please contact us within 2 days after receiving the machine.